| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 6,050 | 6,250 | 18:09 |
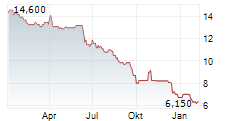
► 09.03.: | | | | | | | | |
► 06.03.: | | | | | | | | |
► Aktueller 4BASEBIO Börsenkurs + Xetra-Orderbuch
► 06.03.: | | | | | | | | |
► Aktueller 4BASEBIO Börsenkurs + Xetra-Orderbuch
| Stück | Geld | Kurs | Brief | Stück |
|---|---|---|---|---|
| 22,200 | 30 | |||
| 19,600 | 2 | |||
| 16,000 | 500 | |||
| 15,000 | 15 | |||
| 6,450 | 69 | |||
| 6,350 | 69 | |||
| 6,250 | 1.861 | |||
Quelle: [URL] https://aktienkurs-orderbuch.finanznachrichten.de/88q.htm [/URL] | ||||
| 715 | 6,100 | |||
| 1.146 | 6,050 | |||
| 69 | 6,000 | |||
| 69 | 5,900 | |||
| 450 | 5,300 | |||
| 1.061 | 0,001 | |||
| Summe Aktien im Kauf | Verhältnis | Summe Aktien im Verkauf | ||
| 3.510 | 0,725 | 2.546 | ||
| Uhrzeit | Aktienkurs | Stück |
|---|---|---|
| 13:04:40 | 6,100 | 1.000 |
| 13:01:26 | 6,100 | 807 |
| 09:04:52 | 6,100 | 50 |
| Tagesumsatz Xetra | -0,100 -1,60 % | 2.857 |
Angaben in grüner Schrift zeigen, dass der Aktienkurs im Vergleich zum vorherigen Kurs gestiegen ist. Angaben in roter Schrift zeigen gefallene Börsenkurse.
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 4BASEBIO Aktie jetzt für 0€ handeln | |||||
| 08:25 | 4basebio promotes Christine Wolosin to chief commercial officer | 4 | Alliance News | ||
| Di | 4basebio strengthens commercial leadership, promoting Christine Wolosin to Chief Commercial Officer | 192 | GlobeNewswire (Europe) | Seasoned biotechnology commercial leader will drive global growth strategy Commercial team further bolstered by promotions of James Vang and Jennifer Gelman to VP, Business Development and VP, Marketing... ► Artikel lesen | |
| Di | 4basebio Plc - Appointment of CCO | 223 | PR Newswire | 4basebio Plc - Appointment of CCO
PR Newswire
LONDON, United Kingdom, March 03
THIS... ► Artikel lesen | |
| 16.02. | 4BASEBIO PLC: Director Dealing | 8 | Cision News | ||
| 16.02. | 4basebio Plc - Director Dealing | 294 | PR Newswire | 4basebio Plc - Director Dealing
PR Newswire
LONDON, United Kingdom, February 16
16 February... ► Artikel lesen |
1 2 3 4 5 Weiter >>
34 Nachrichten in den letzten 12 Monaten
| Zeit | Aktuelle Nachrichten | Medien | ||
|---|---|---|---|---|
| 16:09 | Cathie Wood schlägt wieder zu: Star-Investorin kauft jetzt diese Aktien (BioNTech dabei) | BÖRSE ONLINE | Die Star-Investorin Cathie Wood hat die Schwächephase an den Märkten für weitere Käufe genutzt und eine Reihe spannender Aktien gekauft. Mit dabei ist unter anderem der deutsche Wert BioNTech. Fondsmanagerin... ► Artikel lesen | |
| 13:12 | Sangamo Therapeutics, Inc.: Sangamo Therapeutics Advances Rolling Submission of BLA to U.S. FDA for ST-920 in Fabry Disease | GlobeNewswire (Europe) | Data support potential of isaralgagene civaparvovec as a one-time, well tolerated and durable Fabry disease gene therapy to provide meaningful, multi-organ clinical benefits that could fundamentally... ► Artikel lesen | |
| 13:14 | Coeptis Therapeutics: Vanderbilt Report: Coeptis / Z Squared Merger Enters Final Stretch - Closing Imminent as Institutional Tailwinds Build | ACCESS Newswire | BRISTOL, TN / ACCESS Newswire / March 9, 2026 / When Coeptis Therapeutics Holdings, Inc. (Nasdaq:COEP) and Z Squared Inc. announced their transformational merger in April 2025, the process ahead was... ► Artikel lesen | |
| 13:25 | Gubra A/S: AbbVie reports Positive Phase 1 Multiple Ascending Dose Results for ABBV-295, a Long-Acting Amylin Analog | GlobeNewswire (Europe) | Gubra's partner AbbVie today announced positive topline results from the 12 and 13-weeks Phase 1 Multiple Ascending Dose (MAD) trial showing that ABBV-295 was well tolerated with a dose-dependent significant... ► Artikel lesen | |
| 13:00 | NewcelX Ltd.: NewcelX Announces Strategic Collaboration with Eledon Pharmaceuticals to Advance NCEL-101 Program for Type 1 Diabetes | PR Newswire | Collaboration Integrates Stem-Cell-Derived Islets with Targeted Immune Modulation
ZURICH, Switzerland, March 9, 2026 /PRNewswire/ -- NewcelX Ltd. ("NewcelX") (Nasdaq:... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu 4BASEBIO PLC finden Sie auf Wallstreet Online.