| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
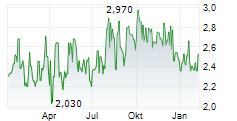
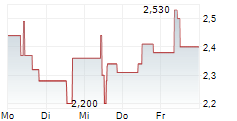
| 2,500 | 2,710 | 07.03. | |
| 2,500 | 2,710 | 06.03. |
► 06.03.: | | | | | | | | |
► 05.03.: | | | | | | | | |
► Aktueller BIOFRONTERA Börsenkurs + Xetra-Orderbuch
► 05.03.: | | | | | | | | |
► Aktueller BIOFRONTERA Börsenkurs + Xetra-Orderbuch
| Stück | Geld | Kurs | Brief | Stück |
|---|---|---|---|---|
| 315,00 | 56.000 | |||
| 150,00 | 10 | |||
| 2,800 | 690 | |||
| 2,710 | 4.277 | |||
Quelle: [URL] https://aktienkurs-orderbuch.finanznachrichten.de/b8fk.htm [/URL] | ||||
| 4.443 | 2,500 | |||
| 1.099 | 0,000 | |||
| Summe Aktien im Kauf | Verhältnis | Summe Aktien im Verkauf | ||
| 5.542 | 11,003 | 60.977 | ||
| Uhrzeit | Aktienkurs | Stück |
|---|---|---|
| 05.03.2026 | 15:41:58 | 2,730 | 1.859 |
| Tagesumsatz Xetra | 0,000 0,00 % | 0 |
Angaben in grüner Schrift zeigen, dass der Aktienkurs im Vergleich zum vorherigen Kurs gestiegen ist. Angaben in roter Schrift zeigen gefallene Börsenkurse.
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | 780 | inv3st.de | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| 17.02. | Biofrontera completes key study for Ameluz skin treatment expansion | 32 | Investing.com | ||
| 14.02. | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | 3.448 | Dow Jones News | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| BIOFRONTERA Aktie jetzt für 0€ handeln | |||||
| 11.02. | Biofrontera's Ameluz SNDA For Superficial Basal Cell Carcinoma Accepted By FDA | 30 | RTTNews | ||
| 09.02. | Biofrontera's Ameluz shows strong results in actinic keratosis trial | 41 | Investing.com |
1 2 3 4 5 Weiter >>
46 Nachrichten in den letzten 12 Monaten
| Zeit | Aktuelle Nachrichten | Medien | ||
|---|---|---|---|---|
| Sa | EQS-Adhoc: Heidelberg Pharma AG: Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von Soleus Capital bekannt | EQS Group (DE) | EQS-Ad-hoc: Heidelberg Pharma AG / Schlagwort(e): Bedeutende Verträge
Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von... ► Artikel lesen | |
| Sa | NurExone präsentiert seine Technologie international - Investoren blicken auf den nächsten Meilenstein | Small- & Micro Cap Investment | ||
| Fr | PMV Pharmaceuticals, Inc.: PMV Pharmaceuticals Reports Full Year 2025 Financial Results and Corporate Highlights | GlobeNewswire (Europe) | Enrollment remains on track in Phase 2 pivotal portion of PYNNACLE trial evaluating rezatapopt as monotherapy in platinum-resistant/refractory ovarian cancer patients with a TP53 Y220C mutationRezatapopt... ► Artikel lesen | |
| Fr | Adial Pharmaceuticals, Inc: Adial Pharmaceuticals Reports 2025 Fiscal Year Financial Results and Provides Business Update | GlobeNewswire (Europe) | GLEN ALLEN, Va., March 06, 2026 (GLOBE NEWSWIRE) -- Adial Pharmaceuticals, Inc. (NASDAQ: ADIL) ("Adial" or the "Company"), a clinical-stage biopharmaceutical company focused on developing therapies... ► Artikel lesen | |
| Fr | Day One Biopharmaceuticals, Inc.: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | GlobeNewswire (Europe) | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs Transaction represents total... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIOFRONTERA AG finden Sie auf Wallstreet Online.