| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
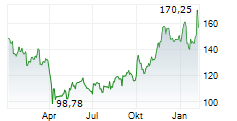
| 159,95 | 160,25 | 07.03. | |
| 158,65 | 159,80 | 06.03. |
► 06.03.: | | | | | | | | |
► 05.03.: | | | | | | | | |
► Aktueller BIOGEN Börsenkurs + Xetra-Orderbuch
► 05.03.: | | | | | | | | |
► Aktueller BIOGEN Börsenkurs + Xetra-Orderbuch
| Stück | Geld | Kurs | Brief | Stück |
|---|---|---|---|---|
| 285,00 | 10 | |||
| 266,00 | 45 | |||
| 244,00 | 35 | |||
| 222,00 | 45 | |||
| 190,00 | 30 | |||
| 185,60 | 5 | |||
| 168,80 | 20 | |||
| 156,90 | 38 | |||
| 156,75 | 376 | |||
Quelle: [URL] https://aktienkurs-orderbuch.finanznachrichten.de/idp.htm [/URL] | ||||
| 376 | 156,30 | |||
| 38 | 156,20 | |||
| 40 | 1,234 | |||
| 1.063 | 0,000 | |||
| Summe Aktien im Kauf | Verhältnis | Summe Aktien im Verkauf | ||
| 1.517 | 0,398 | 604 | ||
| Uhrzeit | Aktienkurs | Stück |
|---|---|---|
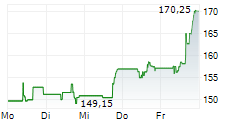
| 06.03.2026 | 17:28:13 | 157,50 | 20 |
| 06.03.2026 | 17:24:34 | 157,30 | 6 |
| 06.03.2026 | 16:40:28 | 158,80 | 11 |
| 06.03.2026 | 11:57:58 | 161,25 | 24 |
| Tagesumsatz Xetra | -5,55 -3,42 % | 90 |
Angaben in grüner Schrift zeigen, dass der Aktienkurs im Vergleich zum vorherigen Kurs gestiegen ist. Angaben in roter Schrift zeigen gefallene Börsenkurse.
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| BIOGEN Aktie jetzt für 0€ handeln | |||||
| Mi | Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | 604 | GlobeNewswire (Europe) | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen | |
| 24.02. | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | 976 | PR Newswire | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| 20.02. | Barclays initiates Biogen stock coverage with $185 price target | 4 | Investing.com | ||
| 20.02. | Barclays startet Biogen-Bewertung mit Kursziel von 185 Dollar | 7 | Investing.com Deutsch | ||
| 20.02. | Biogen und BioArctic: Debatte um Alzheimer-Mittel in Deutschland spitzt sich zu | 488 | Der Aktionär | Im vergangenen Jahr hat Biogen mit seinem japanischen Partner Eisai das Alzheimer-Medikament Leqembi (Lecanemab) auch in Deutschland auf den Markt gebracht. Doch es herrschen Zweifel an der Wirksamkeit... ► Artikel lesen |
1 2 3 4 5 Weiter >>
267 Nachrichten in den letzten 12 Monaten
| Zeit | Aktuelle Nachrichten | Medien | ||
|---|---|---|---|---|
| Sa | EQS-Adhoc: Heidelberg Pharma AG: Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von Soleus Capital bekannt | EQS Group (DE) | EQS-Ad-hoc: Heidelberg Pharma AG / Schlagwort(e): Bedeutende Verträge
Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von... ► Artikel lesen | |
| Sa | NurExone präsentiert seine Technologie international - Investoren blicken auf den nächsten Meilenstein | Small- & Micro Cap Investment | ||
| Fr | Day One Biopharmaceuticals, Inc.: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | GlobeNewswire (Europe) | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs Transaction represents total... ► Artikel lesen | |
| Fr | Tevogen Bio Inc: Letter to Shareholders from CEO Dr. Ryan Saadi | GlobeNewswire (Europe) | WARREN, N.J., March 06, 2026 (GLOBE NEWSWIRE) -- Tevogen ("Tevogen Bio Holdings Inc." or "Company") (Nasdaq: TVGN). Dear Shareholders, As we continue executing on Tevogen's long-term strategy to evolve... ► Artikel lesen | |
| Sa | Mesoblast mit wachsendem Stammzellgeschäft - folgen Eli Lilly und Novartis? | Small- & Micro Cap Investment |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIOGEN INC finden Sie auf Wallstreet Online.